What is PEM Fuel Cell ?
What is PEM fuel Cell? How PEM Fuel Cell works? What are the components of PEM Fuel Cell? A Deep dive into individual components.
FUEL CELL
Deep dive into PEM Fuel Cells
Proton Exchange Membrane (PEM) fuel cells are at the forefront of clean energy technology, offering a promising alternative to traditional fossil fuels. This article provides a comprehensive exploration of PEM fuel cells, delving into their workings, components, and the science behind their operation.
Introduction to PEM Fuel Cells
PEM fuel cells, also known as Polymer Electrolyte Membrane fuel cells, are a type of fuel cell that utilizes a proton-conducting polymer membrane as an electrolyte. They are known for their efficiency, low operating temperatures, and versatility in applications ranging from portable electronics to vehicles and stationary power generation.
How PEM Fuel Cells Work?
PEM fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. The core of the fuel cell consists of the anode, cathode, and the proton-conducting membrane. Here's a step-by-step breakdown of the process:
Hydrogen Supply: Hydrogen gas (H₂) is supplied to the anode side of the fuel cell.
Catalyst Action at Anode: At the anode, a platinum catalyst facilitates the separation of hydrogen molecules into protons (H⁺) and electrons (e⁻).
Proton Conduction: The protons pass through the proton-conducting membrane (electrolyte) to the cathode.
Electron Flow: The electrons flow through an external circuit from the anode to the cathode, generating an electric current.
Oxygen Supply: Oxygen gas (O₂) is supplied to the cathode side.
Catalyst Action at Cathode: At the cathode, another platinum catalyst facilitates the reaction of oxygen molecules with the protons and electrons, producing water (H₂O) as a byproduct.
Electrochemical Reactions
The overall reaction in a PEM fuel cell can be summarized as: 2H2+O2→2H2O+energy2H2+O2→2H2O+energy
Breaking this into half-reactions:
Anode reaction: H2→2H++2e−H2→2H++2e−
Cathode reaction: O2+4H++4e−→2H2OO2+4H++4e−→2H2O
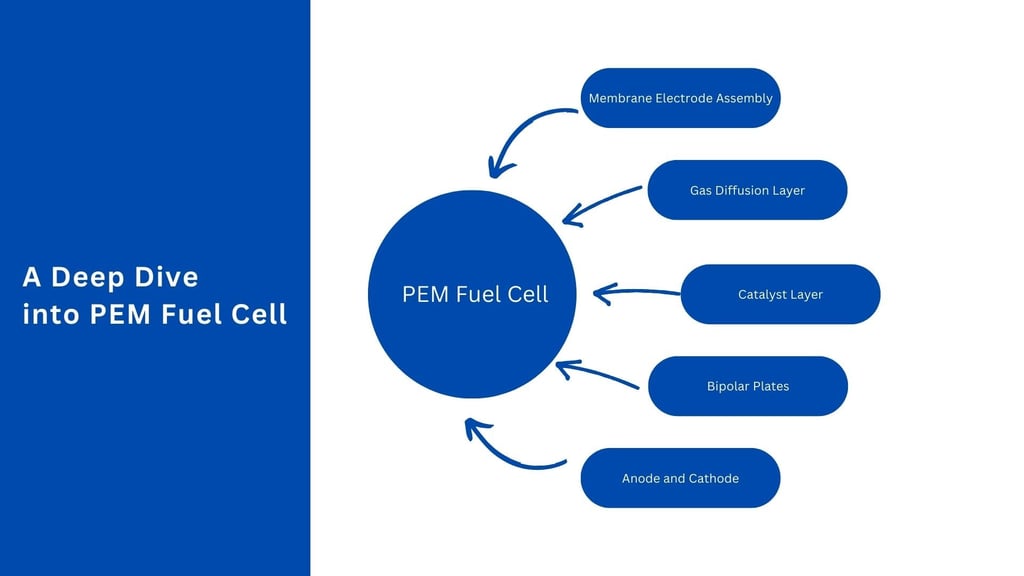
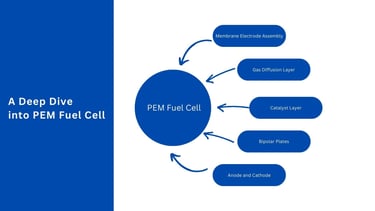
Components of PEM Fuel Cells
A detailed understanding of each component in a PEM fuel cell is crucial for appreciating its operation and design intricacies.
1. Proton Exchange Membrane (PEM)
The PEM is a key component that only allows protons to pass through it, blocking electrons. It’s typically made of a hydrated polymer, such as Nafion. This membrane is crucial for maintaining separation of the reactant gases while facilitating the conduction of protons from the anode to the cathode.
2. Anode
The anode is the electrode where hydrogen gas is introduced. It is typically made of a porous material like carbon paper or carbon cloth, which is coated with a platinum catalyst. The primary functions of the anode are to facilitate the oxidation of hydrogen and to conduct electrons to the external circuit.
3. Cathode
The cathode is the electrode where oxygen gas is introduced. Like the anode, it is made of a porous material coated with a platinum catalyst. The cathode facilitates the reduction of oxygen and the recombination of protons and electrons to form water.
4. Catalyst Layers
Both the anode and cathode contain catalyst layers, usually made of platinum nanoparticles supported on carbon. These layers are crucial for speeding up the electrochemical reactions. The platinum catalyst helps in breaking down the hydrogen molecules at the anode and facilitating the oxygen reduction reaction at the cathode.
5. Gas Diffusion Layers (GDLs)
Gas diffusion layers are placed adjacent to both the anode and cathode. These layers, typically made of carbon fiber paper or cloth, help in the distribution of gases evenly across the catalyst layers, assist in water management, and provide electrical conductivity.
6. Bipolar Plates
Bipolar plates are placed on either side of the membrane-electrode assembly. They serve several functions:
Gas Distribution: Channels in the plates distribute hydrogen and oxygen gases uniformly.
Current Collection: Plates collect and conduct the electric current generated by the fuel cell.
Structural Support: Plates provide mechanical support to the cell stack. Bipolar plates can be made from graphite, metals, or composite materials, each offering different advantages in terms of conductivity, durability, and manufacturing cost.
7. Membrane Electrode Assembly (MEA)
The MEA is the heart of the PEM fuel cell, consisting of the proton exchange membrane sandwiched between the anode and cathode catalyst layers, with gas diffusion layers adjacent to each catalyst layer. The MEA is where the key electrochemical processes occur, and its efficiency largely determines the performance of the fuel cell.
Detailed Working Mechanism
Hydrogen Oxidation at the Anode
Hydrogen gas is introduced to the anode side, where it diffuses through the gas diffusion layer to reach the platinum catalyst. At the catalyst surface, hydrogen molecules are split into protons and electrons: H2→2H++2e−H2→2H++2e− The protons pass through the PEM to the cathode, while the electrons are conducted through the external circuit, creating an electric current.
Proton Conduction Through the Membrane
The PEM, typically Nafion, is a hydrated polymer that conducts protons effectively. It blocks electrons, forcing them to travel through the external circuit. The efficiency of proton conduction is critical and depends on the membrane's hydration level and temperature.
Oxygen Reduction at the Cathode
Oxygen gas is introduced to the cathode side, where it diffuses through the gas diffusion layer to reach the platinum catalyst. At the catalyst surface, oxygen molecules react with protons arriving from the PEM and electrons arriving via the external circuit: O2+4H++4e−→2H2OO2+4H++4e−→2H2O This reaction forms water as a byproduct, which must be managed to prevent flooding of the catalyst sites and gas diffusion layers.
Water and Thermal Management
Efficient water management is crucial in PEM fuel cells. The produced water must be removed to prevent flooding but retained enough to keep the membrane hydrated. This balance is typically managed through a combination of gas flow management, humidification of reactant gases, and temperature control.
Temperature management is equally important. While PEM fuel cells operate at relatively low temperatures (60-80°C), excess heat generated by the reactions must be dissipated to maintain optimal operating conditions and ensure the longevity of the components.
Applications of PEM Fuel Cells
PEM fuel cells are versatile and can be used in various applications, including:
1. Transportation
PEM fuel cells are used in fuel cell electric vehicles (FCEVs), offering advantages such as quick refueling and longer ranges compared to battery electric vehicles (BEVs). They are used in cars, buses, and even trains.
2. Portable Power
PEM fuel cells provide clean and efficient power for portable electronics, backup power systems, and remote power generation.
3. Stationary Power Generation
PEM fuel cells are used in stationary power generation for residential, commercial, and industrial applications, providing reliable and clean energy with potential for grid independence.
4. Backup Power
Due to their quick startup and reliable operation, PEM fuel cells are ideal for backup power systems in critical infrastructure such as data centers, hospitals, and telecommunications.
Challenges and Future Directions
While PEM fuel cells offer numerous advantages, they face several challenges:
1. Cost
The high cost of platinum catalysts and membrane materials remains a significant barrier to widespread adoption. Research is focused on finding cheaper and more abundant catalyst materials, such as platinum alloys or non-platinum alternatives.
2. Durability
The durability of PEM fuel cells is affected by factors such as catalyst degradation, membrane durability, and water management issues. Advances in materials science and engineering are being pursued to enhance the longevity of these cells.
3. Hydrogen Production and Infrastructure
The availability and production of hydrogen are critical. Most hydrogen is currently produced from natural gas, which is not entirely sustainable. Development of green hydrogen production methods, such as electrolysis using renewable energy, and the establishment of hydrogen refueling infrastructure are essential for the future of PEM fuel cells.
4. Water Management
Efficient water management within the cell remains a technical challenge. Innovations in membrane technology and system design are needed to maintain optimal hydration levels and prevent flooding.
Conclusion
PEM fuel cells represent a powerful and versatile technology for the future of clean energy. Their ability to efficiently convert hydrogen and oxygen into electricity, with water as the only byproduct, makes them an attractive solution for reducing carbon emissions and dependence on fossil fuels. As research and development continue to address the challenges of cost, durability, and infrastructure, PEM fuel cells are poised to play a critical role in the global energy landscape.
By understanding the intricate workings and components of PEM fuel cells, we can appreciate their potential and contribute to their advancement, paving the way for a sustainable and greener future.
Interested in buying fuel cells : Explore our products
